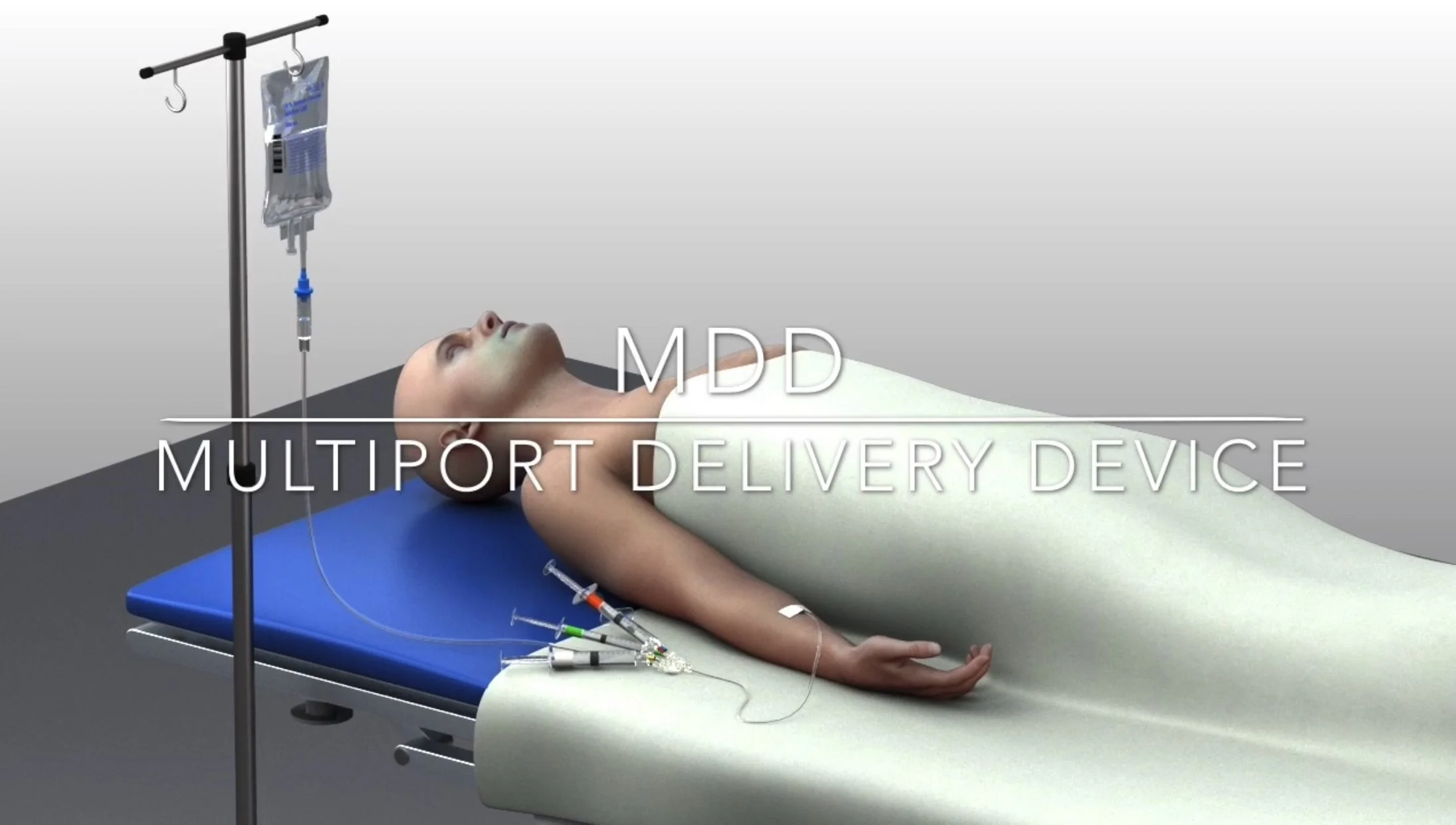
Multiport Delivery Device™ *
U.S. Patent 10,369,272
Our novel Multiport Delivery Device™ (MDD™) is designed to improve compliance and eliminates harmful contaminants from entering a patient’s vascular bloodstream system.
More than 300 million IVs are placed each year in the US and collectively these vascular access lines are accessed more than 2 billion times. In the surgical setting, anesthesiologists will administer a combination of medications to induce a balanced state of pain-free unconsciousness. Each medication is drawn up manually from a glass vial, transferred into a syringe, or provided in a prefilled syringe. The collective syringes are then administered in a pre-planned combination to achieve the anesthetized state. During this quick process, quality of care, safety among practice, and efficiency within performance encompass perioperative protocol. Strategies to prevent intravenous line contamination and bloodstream infection are not always prioritized or practiced. Currently, in healthcare, no device addresses all the potential pitfalls that come with administering multiple IV medications to anesthesia-setting patients.
The MDD™ device has a multi-function purpose purging air, and filtering particulates. In the perioperative environment multi IV drugs are administered to the patient throughout the procedure. The various features of the MDD device provide comprehensive benefits. The self-priming feature purges all air molecules from the IV line that may have been pushed by an anesthesia member or are present within a pre-filled syringe. The multi-port feature allows the cocktail of anesthesia fluids to be delivered through designated induction ports. These designated induction ports are permanently bonded to zero reflux needleless connectors that prevent retrograde mixing of medications and additionally prevent the ingress of bacteria from freely entering the fluid pathway. The fluid pathway contains an integrated dual-filter design to filter pharmaceutical fluid particulates, entrapped air, and the contamination of microbes. Dual check valves maintain a regulated pressure within the device, minimizing precipitates and allowing anesthesia to deliver meds with product compliance.
The Multiport Delivery Device™ (MDD™) is a proprietary and patented device that combines multiple IV ports with at least two filters for variable fluid viscosity in an inline configuration for high flow input/output — all regulated by a high-pressure check valve system.
Simplified Infusion Workflow
Aseptic preparation of medications prior to connection to IV lines
Closure to ambient airflow to reduce sources of microbial ingress
Multiport access for simultaneous, multiple administration of IV medication
Workflow efficiency, safety, and reducing medication waste
Focused on Improving Clinical Outcomes*
Anti-reflux valves to prevent retrograde fluid medication flow
Anti-siphon prevention of retrograde blood flow
Variable viscosity fluid filtration (lipid/non-lipid), particulates, as well as microorganisms
Air Elimination